Vitamin D
Initial Problem
In June 2016, in response to an unmanageable rise in vitamin D requests from 4559 in 2013-2014 to 5669 in 2014-2015 and 8724 in 2015-2016, the Biochemistry department took the slightly drastic step of temporarily halting all vitamin D testing while it sought advice from colleagues in rheumatology and endocrinology about the clinical situations in which measurement was clinically warranted.
Change in practise
Users were issued with the following information:
“Vitamin D testing will be suspended, pending the production and implementation of new requesting guidelines. However its future availability will be severely restricted and it will no longer be available to users in primary care.”
Consultants in rheumatology, endocrinology, paediatrics and care of the elderly rapidly developed draft guidelines specifying the clinical situations in which they required vitamin D to be measured, with more general guidelines indicating that any patient at high risk of being vitamin D deficient should receive supplementation (with OTC preparations rather than prescribed) without measuring vitamin D. After starting supplements, calcium and phosphate should be monitored 10-12 weeks after starting and vitamin D measured only in patients who fail to respond.
Other criteria for measuring vitamin D included patients with primary hyperparathyroidism and complex nutrition patients.
2 weeks after suspension of testing we were able to restart with IT rules in place to block any request that did not come from a rheumatology, endocrinology, care of the elderly or paediatric consultant.
In Cyberlab (our electronic requesting system) requestors from primary care received an automatic message stating “Vitamin D may no longer be requested from primary care.” Requestors from secondary care who were not on the agreed consultant list received a message stating “Vitamin D measurement is currently only available for endocrinology, rheumatology and care of the elderly consultants.” Additional rules were in place in the LIMS to apply the same rules to paper requests.
Impact of change
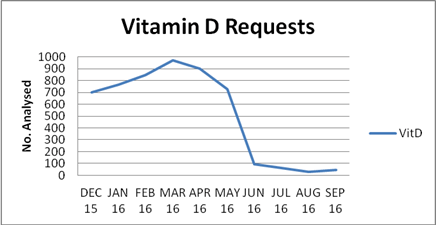
The immediate effect on workload was significant with a drop in requests from an average 840 a month in the 6 months leading up to the intervention to 45 a month in the following 3 months. That decrease has been sustained with annual requests standing at 550 for the year 2017-2018.
More recently secondary care requesting has switched from Cyberlab to TrakCare. In TrakCare requestors receive the following message if vitamin D is requested “Vitamin D should only be requested by the following specialties: Endocrinology, Rheumatology, Paediatrics, Care of the Elderly, or as part of assessment of complex nutritional needs. Cancel this request if it doesn’t meet these requirements.” They are then asked to select the appropriate specialism along with providing clinical information. The rules remain in the LIMS to guard against requesting creep.
There has been very little negative feedback from laboratory users. Most GPs who have spoken to us have been happy not to have to organise yet another test and to accept our proposal that any patients presenting particular concerns about vitamin D status should be referred to rheumatology or endocrinology for investigation if appropriate.
Health Board of Implementation
NHS Fife
Contact
Dr Philip Wenham, Dr Heather Holmes (direct point of contact now for Fife)
